Serialization
Counterfeit medicines are a global problem. According to estimates by health authorities, around 10% of all medicines in circulation worldwide are counterfeit or diluted. At best, the substances are harmless, but they are often harmful to health or even life-threatening.
The EU has therefore imposed requirements for reliable serialization and labeling systems in the pharmaceutical industry in order to combat the illegal trade in counterfeit medicines.
Tamper-proof system from the manufacturer to the pharmacy
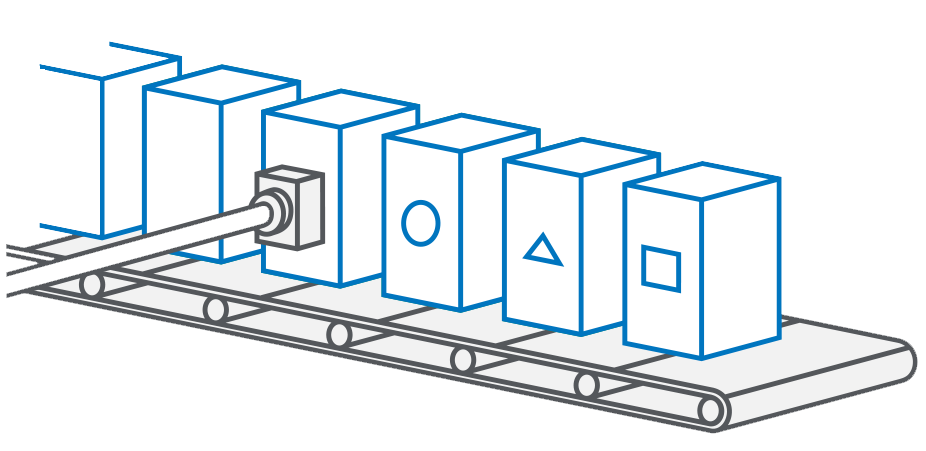
The main feature for the counterfeit protection of a medicine is the allocation of unique serial numbers, which are only available once and allow each individual pack to be reliably checked. In conjunction with individual production data (serialization) and first-opening protection (tamper evidence), this ensures the authenticity and integrity of the medication and enables seamless traceability.
Each pack is given its own unique serial number, which is applied during the manufacturing process and integrated into an end-to-end authenticity verification system. In the pharmacy, each individual pack is verified before it is dispensed to the patient.
Overcoming complex challenges together
Implementing the legal requirements is a complex process for everyone involved. We at db | bauer packaging have built up extensive expertise in this area. We successfully develop and manage serialization in close cooperation with our customers from the pharmaceutical industry.
We are happy to share our know-how with you.
Druckerei Bauer GmbH
Otto-Rettenmaier-Str. 5-6
74629 Pfedelbach
T +49 (7941) 91 74 - 0
info@bauer-packaging.de